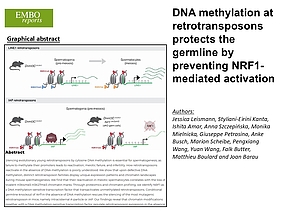
New publication by the Barau lab on how DNA methylation at retrotransposons protects the germline by preventing NRF1-mediated activation
Leismann J, Kanta SE, Amar I, Szczepińska A, Mielnicka M, Petrosino G, Busch A, Scheibe M, Wang P, Wang Y, Butter F, Boulard M, Barau J (2025) DNA methylation at retrotransposons protects the germline by preventing NRF1-mediated activation. EMBO Rep, 26(15):7185 Link
Abstract:
Silencing evolutionary young retrotransposons by cytosine DNA methylation is essential for spermatogenesis, as failure to methylate their promoters leads to reactivation, meiotic failure, and infertility. How retrotransposons reactivate in the absence of DNA methylation is poorly understood. We show that upon defective DNA methylation, distinct retrotransposon families display unique expression patterns and chromatin landscapes during mouse spermatogenesis. We find that their reactivation in meiotic spermatocytes correlates with the loss of bivalent H3K4me3-H3K27me3 chromatin marks. Through proteomics and chromatin profiling, we identify NRF1 as a DNA methylation-sensitive transcription factor that transactivates unmethylated retrotransposons. Conditional germline knockout of Nrf1 in the absence of DNA methylation rescues the silencing of the most mutagenic retrotransposon in mice, namely Intracisternal A-particle or IAP. Our findings reveal that chromatin modifications together with a DNA methylation-sensitive transcription factor regulate retrotransposon expression in the absence of DNA methylation in spermatogenesis, revealing a mechanism by which retrotransposons proliferate in the germline after evading DNA methylation-based silencing.
Read the full paper here: https://www.embopress.org/doi/full/10.1038/s44319-025-00526-1#abstract